Abstract
Background: Venetoclax (Ven), a highly selective BCL-2 inhibitor, combined with azacitidine (Aza) suppresses oxidative phosphorylation, which selectively targets leukemia stem cells that drive initiation and perpetuation of acute myeloid leukemia (AML; Pollyea. Nat Med. 2018;24:1859), an aggressive malignancy most common in older adults. Safety and efficacy of Ven combined with the hypomethylating agents (HMA) Aza or decitabine (Dec) for the treatment of newly diagnosed AML in patients (pts) who are ineligible to receive intensive chemotherapy has been demonstrated (DiNardo. Blood. 2019;133:7; DiNardo. N Engl J Med. 2020;383:617). Phase 1 and Phase 3 studies initiated Ven + HMA in an inpatient setting due to the nature of the study design and concerns of tumor lysis syndrome (TLS) based on chronic lymphocytic leukemia treatment with Ven. Safety and efficacy of Ven + HMA treatment initiation in an exclusively outpatient setting is being evaluated in an ongoing Phase 3b, single-arm, multicenter, open-label study (NCT03941964). Here, we present pt baseline (BL) characteristics and safety during initial outpatient dose ramp-up of Ven + HMA.
Methods: Pts with untreated AML with an ECOG performance status of 0-3 who were ineligible to receive intensive chemotherapy, had no evidence of spontaneous TLS at BL, and deemed an appropriate candidate for outpatient initiation of Ven + HMA by the investigator were eligible. Enrolled pts received Ven (100 mg on Cycle [C] 1 Day [D] 1, 200 mg C1D2, 400 mg C1D3-D28, and 400 mg daily for each 28-day cycle thereafter) in combination with Aza (75 mg/m 2 intravenously [IV] or subcutaneously for 7 days) or Dec (20 mg/m 2 IV for 5 days), beginning on D1 of each cycle, as per institutional practice, for ≤6 cycles. After the study period ended, pts could continue receiving commercially acquired standard-of-care treatments with Ven and Aza or Dec. Ven dosing was modified for concomitant use with moderate and strong CYP3A inhibitors (CYP3Ai). Ven and HMA dosing adjustments were permitted for the management of adverse events (AEs). TLS prophylaxis was initiated in all pts before the first dose of study drug or first new escalated dose. Pts were screened for BL TLS markers. BL AML characteristics, such as blast count and cytogenetics, were examined. The incidence of TLS per Howard Criteria (Howard. N Engl J Med. 2011;364:1844) was assessed for 5 days starting after the first dose of Ven.
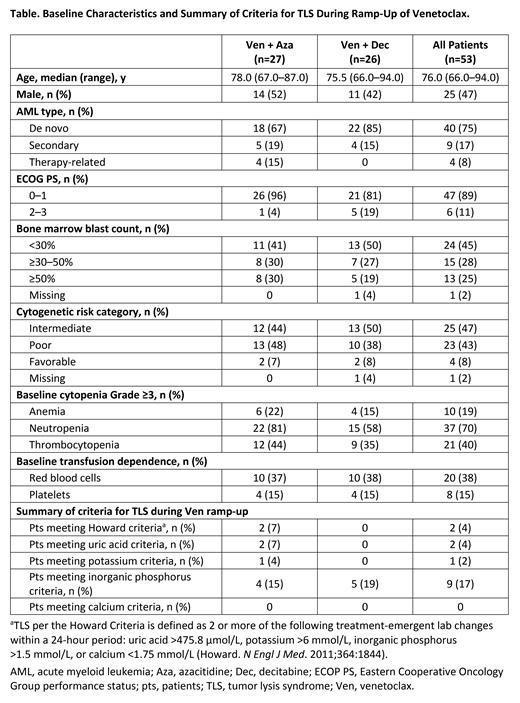
Results: At the data cutoff (April 30, 2021), 53 pts were enrolled, with 27 receiving Ven + Aza and 26 receiving Ven + Dec (Table). Among all pts, the median age was 76 years (range, 66-94), 89% of pts had an ECOG performance status of 0-1, and 60% were ineligible for standard induction therapy due to age ≥75 years. Overall, 15% and 38% of pts were transfusion-dependent on platelets or red blood cells, respectively. Grade 4 neutropenia was present at BL in 51% of pts; renal and hepatic impairment were present at BL in 79% and 19%, respectively.
Most pts had intermediate (47%) or poor (43%) cytogenetic risk. De novo AML was the most common type of disease (75%), followed by secondary (17%) and therapy-related AML (8%). Thirteen pts (25%) had ≥50% bone marrow blasts, and the median blast count was 30% (range, 0-90). In some pts, mutations in biomarkers of interest (FLT3, IDH1/2, NPM1, and TP53) were detected; an analysis will be presented.
Nineteen pts (36%) received prophylactic anti-infective moderate or strong CYP3Ai. During Ven ramp-up, there were no cases of clinical TLS and 2 cases (4% of pts) of laboratory TLS; both pts were hospitalized and managed with dose interruption and medical intervention, and neither case led to treatment or study discontinuation. Three pts were hospitalized during Ven ramp-up for other serious AEs.
Conclusion: In our study of pts receiving Ven + HMA in an outpatient setting at US community-based oncology centers, most had an ECOG performance status of 0-1 and were not platelet or red blood cell transfusion-dependent. Disease was primarily de novo AML, and 74% of pts had bone marrow blast counts <50%. During ramp-up of Ven, <10% of pts required hospitalization for any reason; 4% of pts met criteria for TLS, without requiring discontinuation. These results indicate that, with appropriate TLS prophylaxis and monitoring, pts with untreated AML can safely initiate Ven in an outpatient setting.
Manda: Genmab: Current equity holder in publicly-traded company; Morphosys: Honoraria. Benton: AbbVie: Consultancy; Karyopharm: Consultancy; Pharmaessentia: Consultancy. Yimer: Astrazeneca: Speakers Bureau; Janssen: Speakers Bureau; Karyopharm: Current equity holder in publicly-traded company, Speakers Bureau; Beigene: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Speakers Bureau; Pharmacyclics: Speakers Bureau; Texas Oncology: Current Employment. Renshaw: Amgen: Speakers Bureau; SeaGen: Consultancy; Jazz Pharmaceuticals: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau; Texas Oncology: Current Employment. Geils: Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Janssen Oncology: Honoraria, Speakers Bureau. Fanning: Sanofi: Speakers Bureau; TG Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genmab: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; BMS: Speakers Bureau. Melear: Astrazeneca: Speakers Bureau; TG Therapeutics: Speakers Bureau; Janssen: Speakers Bureau. Sharman: BMS: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; TG Therapeutics: Consultancy; BeiGene: Consultancy; Lilly: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; Centessa: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Kang: AbbVie: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Svensson: AbbVie: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Pai: AbbVie: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal